Answer:
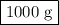
Step-by-step explanation:
We know we will need a balanced equation with masses, moles, and molar masses, so let’s gather all the information in one place.
M_r: 84.01
H₂SO4 + 2NaHCO₃ ⟶ Na₂SO₄ + 2CO₂ + 2H₂O
n/mol: 6
1. Use the molar ratio of NaHCO₃ to calculate the moles of NaHCO₃.
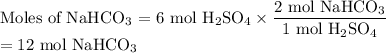
2. Use the molar mass of NaHCO₃ to calculate the mass of NaHCO₃.
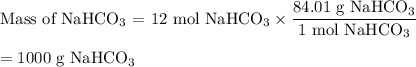
You must use
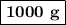 of NaHCO₃.
of NaHCO₃.