Hello!
The answer is:
The new temperature of the gas is equal to 388.22 K
Why?
Since we have a rigid container, we can safely assume that the volume is kept constant.
To solve the problem, we need to use the Gay Lussac's Law, which states that the pressure and the volume of an ideal gas are proportional when the volume is kept constant.
Also, we need to remember that Gay Lussac's Law works with absolute temperature, so, we need to convert the given temperature (in celsius degrees) to Kelvin.
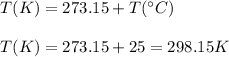
So, using the Gay Lussac's equation, we have:
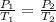
Where,
 is equal to the first pressure.
is equal to the first pressure.
 is equal to the first temperature.
is equal to the first temperature.
 is equal to the new pressure.
is equal to the new pressure.
 is equal to the new temperature.
is equal to the new temperature.
We are given the following information:
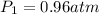
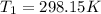
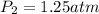
Then, substituting and calculating, we have:
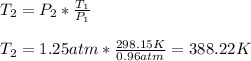
Hence, the new temperature of the gas is equal to 388.22 K.
Have a nice day!