Answer:
The gas with the highest average velocity is a) H2
Step-by-step explanation:
The average kinetic energy is a value that depends on the temperature of the gas. Because of H2,He and N2 are at the same temperature their average kinetic energy will be the same.
For the average velocity, the equation is :
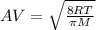
Where R is the Universal gas constant.
Where T is the temperature.
And M is the molar mass of the gas.
Given that M is dividing the expression, the gas with the highest average velocity will be the gas with the smallest molar mass.
The molar masses of the gases are :
μ(H2) =
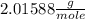
μ(He) =
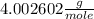
μ(N2) =
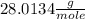
H2 has the lowest molar mass. Therefore, H2 will have the highest average velocity.