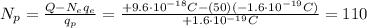Answer:
110
Step-by-step explanation:
The net charge of the compound is the sum of the net charges due to the electrons and the protons:
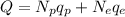
where
Np is the number of protons
Ne = 50 is the number of electrons
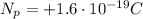 is the charge of each proton
is the charge of each proton
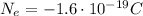 is the charge of each electron
is the charge of each electron
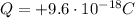 is the net charge of the compound
is the net charge of the compound
Solving the equation for Np, we find the number of protons: