Answer:
c) Because light is packaged discretely, increasing the intensity of light will increase the number of photons hitting a metal and thus the likelihood that an electron will be ejected.
Step-by-step explanation:
In the photoelectric effect, light incident with a certain frequency causes the emission of photoelectrons from the surface of a metal. This effect is explained by considering light a "package" of several quanta, called photons: each photon hits only 1 electron at time, giving all its energy to the electron. If the energy given is above the work function, then the electron has enough energy to leave the metal.
The energy given off by the photon only depends on the frequency of the light, not on the intensity, according to the formula
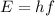
where h is the Planck constant and f the frequency.
In this model, one photon hits only 1 electron at time: this means that the intensity of light (which is a measure of the number of photons in the light) does not affect the probability of emitting an electron from the material, because that probability depends only on the energy of the photon, which depends only on the frequency of the light.
So, statement c) is wrong.