Answer:
b. AG, work function=4.74eV
Step-by-step explanation:
Ultraviolet light starts at the end of the visible light spectrum, where violet light ends:
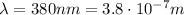 (wavelength of lowest-energy ultraviolet light)
(wavelength of lowest-energy ultraviolet light)
So, the lowest energy of ultraviolet light can be found by using the formula

where
h is the Planck constant
c is the speed of light
Substituting,
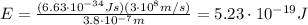
And keeping in mind that
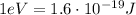
This energy converted into electronvolts is
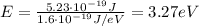
The work function of a metal is the minimum energy needed to extract a photoelectron from the surface of the metal. Therefore, the metals that exhibit photoelectric effect are the ones whose work function is larger than the energy we found previously, so:
b. AG, work function=4.74eV
Because for all the other metals, visible light will be enough to extract photoelectrons.