Answer: The amount of substance left will be 0.316 grams.
Step-by-step explanation:
All the decay processes follow first order kinetics.
The equation used to calculate half life for first order kinetics:
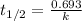
where,
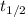 = half life of the reaction = 14 days
= half life of the reaction = 14 days
k = ?
Putting values in above equation, we get:
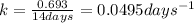
Rate law expression for first order kinetics is given by the equation:
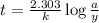
where,
k = rate constant =
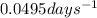
t = time taken for decay process = 70 days
a = initial amount of the reactant = 10 grams
y = amount left after decay process = ? grams
Putting values in above equation, we get:
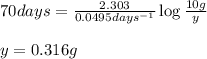
Hence, the amount of substance left will be 0.316 grams.