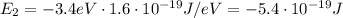Answer:
-3.4 eV (
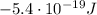 )
)
Step-by-step explanation:
In a hydrogen atom, the energy of an electron is given by:
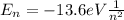
where
n is the principal quantum number
For an electron in the 2s orbital,
n = 2
So substituting this value into the formula, we find
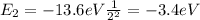
And converting this into Joules, we have