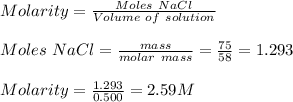Answer:
1) [OH-] = 1*10⁻¹⁰ mol/L
Solution is Acidic
2) V = 14.4 L
3) q = -26125 J
4) Concentration of NaCl = 2.59 M
Step-by-step explanation:
1) Given:
[H+] = 1*10⁻⁴ mol/L
Formula:
![[H+][OH-] = 10^(-14) \\](https://img.qammunity.org/2020/formulas/chemistry/middle-school/s1fqk7edfyj2zg2tu4ejuz4rlp7k2989c0.png)
![[OH-] = (10^(-14) )/(10^(-4) ) \\\\[OH-] = 1*10^(-10) mol/L](https://img.qammunity.org/2020/formulas/chemistry/middle-school/1ez8ompmqb59gapxdgrm2nkf7gk3kdfy0u.png)
![p[H] = -log[H+] = -log[10^(-4) ] = 4](https://img.qammunity.org/2020/formulas/chemistry/middle-school/lsy39cofxp46in99u836h5mlirg0w4hmcj.png)
Since pH < 7, the solution is acidic
2) Given:
Initial conditions:
Pressure, P1 = 1 atm
Temperature, T1 = 273 K
Volume, V1 = 10.0 L
Final conditions:
Pressure, P2 = 2.0 atm
Temperature, T2 = 512+273 = 785 K
Volume, V2 = ?
Formula:
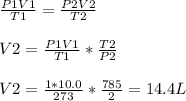
3) Given:
Volume of tea = 250 ml
Initial temp T1 = 375 K
Final temp, T2 = 350 K
Formula:
Energy transferred, q = mcΔT = mc(T2-T1)
m = mass of tea (water) = density * volume = 1 g/ml * 250 ml = 250 g
c = specific heat of tea (water) = 4.18 J/ gK
ΔT = T2-T1 = 350-375 = -25 K
q = 250*4.18*(-25) = -26125 J
4) Given:
Volume of sea water = 0.500 L
Mass of NaCl = 75 g
Molar mass of NaCl = 58 g/mol
Formula: