Answer:
0.048M
Step-by-step explanation:
Volume of acid = 25mL = 0.025L
Concentration of base NaOH = 0.12M
Volume of base NaOH = 10mL= 0.01L
Unknown:
concentration or molarity of the nitric acid = ?
Solution
We first write the balanced reaction equation:
HNO₃ + NaOH → NaNO₃ + H₂O
In this problem, the focus is on the reacting acid and base which are on the left hand side.
Now, to find the molarity of the nitric acid that would be completely neutralized by the base, we adopt the mole concept.
First, we work from the known to the unknow. We can find the number of moles of the base given because its parameters are complete. The number of moles is given by the expression below:
Number of moles = concentration x volume
To find the number of moles of the base, we put the parameters into the expression above:
Number of moles of NaOH = concentration of NaOH x volume of NaOH
= 0.01 x0.12
= 0.0012mol
From the number of moles of the base, we can find the unknown.
In the reaction equation:
1 mole of base neutralizes 1 mole of the acid
0.0012mole of the base will neutralize 0.0012mole of the acid
Therefore, 0.0012mole of HNO₃ was neutralized.
Now we can calculate the molarity of the acid using the expression below:
concentration of acid =
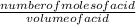
concentration of acid =
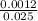 = 0.048M
= 0.048M