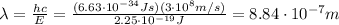Answer:
Step-by-step explanation:
The energy a single photon of an electromagnetic wave is given by

where
h is the Planck constant
c is the speed of light
 is the wavelength of the photon
is the wavelength of the photon
In this problem, we have
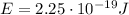 is the energy of the photon
is the energy of the photon
So we can re-arrange the equation to find the wavelength: