Answer: B) 1 Calorie
Step-by-step explanation: The heat required to raise the temperature of 1 gram of a substance by 1 degree C is known as its specific heat capacity.
Specific heat capacity of water is
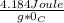 and we know that, 4.184 Joule = 1 Calorie
and we know that, 4.184 Joule = 1 Calorie
So, specific heat capacity of water is
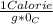 and hence the correct choice is B.
and hence the correct choice is B.