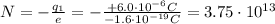A) 16.1 N
The magnitude of the electric force between the corks is given by Coulomb's law:
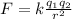
where
k is the Coulomb's constant
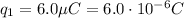 is the magnitude of the charge on the first cork
is the magnitude of the charge on the first cork
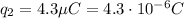 is the magnitude of the charge of the second cork
is the magnitude of the charge of the second cork
r = 0.12 m is the separation between the two corks
Substituting numbers into the formula, we find
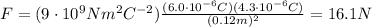
B) Attractive
According to Coulomb's law, the direction of the electric force between two charged objects depends on the sign of the charge of the two objects.
In particular, we have:
- if the two objects have charges with same sign (e.g. positive-positive or negative-negative), the force is repulsive
- if the two objects have charges with opposite sign (e.g. positive-negative), the force is attractive
In this problem, we have
Cork 1 has a positive charge
Cork 2 has a negative charge
So, the force between them is attractive.
C)
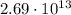
The net charge of the negative cork is
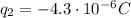
We know that the charge of a single electron is
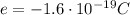
The net charge on the negative cork is due to the presence of N excess electrons, so we can write
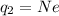
and solving for N, we find the number of excess electrons:
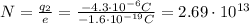
D)
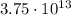
The net charge on the positive cork is
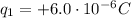
We know that the charge of a single electron is
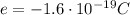
The net charge on the positive cork is due to the "absence" of N excess electrons, so we can write
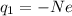
and solving for N, we find the number of electrons lost by the cork: