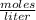Answer:
The molarity of 500.0 grams of Li₂S in a total volume of 4.44 liters is 2.448
Step-by-step explanation:
Molarity (M) is the number of moles of solute (substance that dissolves in a solvent with which it forms a solution, is in a smaller proportion) that are dissolved in a given volume.
Molarity is expressed by the following expression:
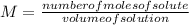
Molarity is expressed in units
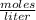
Then, you must first know the amount of moles that represent the 500 g of Li₂S. For that, a rule of three is applied knowing the molar mass: if 46 g is 1 mol of Li₂S, 500 g of the compound, how many moles are they?

moles≅10.87
It is now possible to calculate molarity by applying the definition:
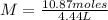
M= 2.448
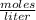
The molarity of 500.0 grams of Li₂S in a total volume of 4.44 liters is 2.448