Answer:
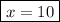
Step-by-step explanation:
This is like an empirical formula question, except that you are finding the molar ratio of compounds instead of atoms.
Step 1. Gather the information in one place.
M_r: 142 18
Na₂SO₄·xH₂O(s) ⟶ Na₂SO₄(s) + xH₂O(g)
m/g: 3.22 1.42
Step 2. Calculate the mass of the water
Mass of H₂O = mass of Na₂SO₄·xH₂O – mass of Na₂SO₄
= 3.22 – 1.42 = 1.80 g
Step 3. Calculate the moles of each product
Na₂SO₄:
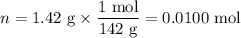
H₂O:
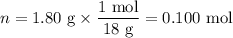
Step 4. Calculate the molar ratios
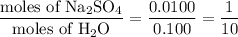
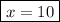 , so the formula of the compound is Na₂SO₄·10H₂O
, so the formula of the compound is Na₂SO₄·10H₂O