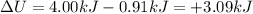Answer:
3. +3.09 kJ
Step-by-step explanation:
The change in internal energy of the gas is given by the 1st law of thermodynamics:
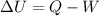
where
Q is the heat transferred to the gas
W is the work done by the gas
Here we have:
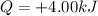 is the amount of heat transferred to the nitrogen
is the amount of heat transferred to the nitrogen
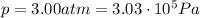 is the pressure of the gas
is the pressure of the gas
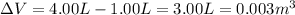 is the change in volume of the gas
is the change in volume of the gas
So the work done by the gas is
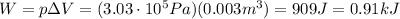
So, the change in internal energy of the gas is