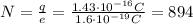Answer:
894 electrons
Step-by-step explanation:
The electrostatic force between the two charges is given by:
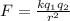
where we have
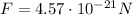 is the force
is the force
k is the Coulomb's constant
q1 = q2 =q is the magnitude of the charge on each sphere
r = 20.0 cm = 0.20 m is the distance between the two spheres
Substituting and solving for q, we find the charge on each sphere:
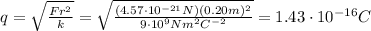
And since each electron has a charge of
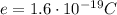
the net charge on each sphere will be given by
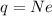
where N is the number of excess electrons; solving for N,