Answer:
It is directly proportional
Step-by-step explanation:
For an ideal gas, the average kinetic energy of the particles and the temperature (in Kelvin) are directly proportional, according to the equation:
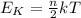
where
Ek is the average kinetic energy
k is the Boltzmann constant
T is the absolute temperature
n is the number of degrees of freedom of the molecules in the gas (n=3 for monoatomic gases, n=5 for di-atomic gases, etc..)
Therefore, as the temperature of a gas increases, the average kinetic energy of the molecules increases proportionaly to it.