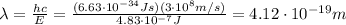(a)
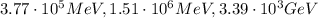
The energy levels of an electron in a box are given by
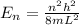
where
n is the energy level
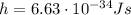 is the Planck constant
is the Planck constant
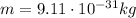 is the mass of the electron
is the mass of the electron
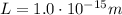 is the size of the box
is the size of the box
Substituting n=1, we find the energy of the ground state:
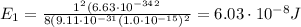
Converting into MeV,
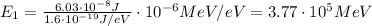
Substituting n=2, we find the energy of the first excited state:
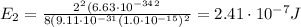
Converting into MeV,
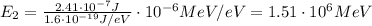
Substituting n=3, we find the energy of the second excited state:
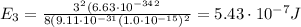
Converting into GeV,
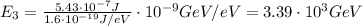
(b)
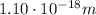
The energy of the emitted radiation is equal to the energy difference between the two levels, so:
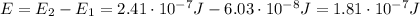
And the energy of the electromagnetic radiation is
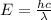
where c is the speed of light; so, re-arranging the formula, we find the wavelength:
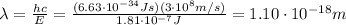
(c)
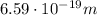
The energy of the emitted radiation is equal to the energy difference between the two levels, so:
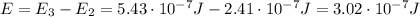
Using the same formula as before, we find the corresponding wavelength:
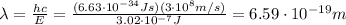
(d)
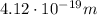
The energy of the emitted radiation is equal to the energy difference between the two levels, so:
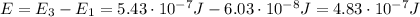
Using the same formula as before, we find: