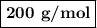Answer:
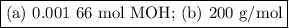
Step-by-step explanation:
I am assuming that the unknown acid is monobasic. Then the general equation for the reaction is
HA + MOH ⟶ MA + H₂O
(a) Moles of MOH
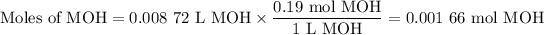
(b) Moles of HA
Now, you use the molar ratio from the balanced chemical equation to find the moles of unknown acid.
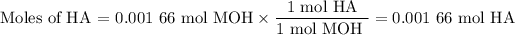
(c) Molar mass of HA
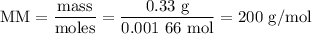
The molar mass of the unknown acid is