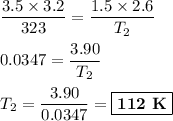Answer:
Step-by-step explanation:
A) Know
p₁ = Initial pressure; p₂ = final pressure
V₁ =Initial volume; V₂ = final pressure
T₁ = Initial temperature
B) Find
T₂ = final temperature
C) Strategy
Use the Combined Gas Laws Equation:
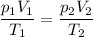
It contains symbols for all the knowns and the unknown.
D) Calculations
(i) Data:
p₁ = 3.5 atm; p₂ = 1.5 L
V₁ = 3.2 L; V₂ = 2.6 L
T₁ = 323 K T₂ = ?
(ii) Calculation