Answer:
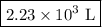
Step-by-step explanation:
The pressure is constant, so we can use Charles' Law.
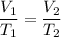
Data:
V₁ = 1.92 × 10³ L; T₁ = 20 °C
V₂ = ?; T₂ = 68 °C
Calculations:
(a) Convert temperatures to kelvins
T₁ = (20 + 273.15) K = 293.15 K
T₂ = (68 + 273.15) K = 341.15 K
(b) Calculate the volume
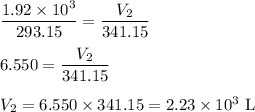
The new volume of the gas is
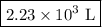 .
.