Answer:
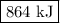
Step-by-step explanation:
The formula for the heat transferred to or from an object is
q = mCΔT
Data:
m = 2.94 kg
C = 1.75 J·°C⁻¹g⁻¹
T₁ = 23 °C
T₂ = 191 °C
Calculations:
(a) Convert the mass to grams
m = 2.94 kg = 2940 g
(b) Calculate ΔT
ΔT = T₂ - T₁ = 191 – 23 = 168 °C
(c) Calculate q
q = 2940 × 1.75 × 168 = 864 000 J = 864 kJ
You must provide
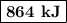 .
.