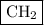Answer:
Step-by-step explanation:
1. Calculate the mass of each element
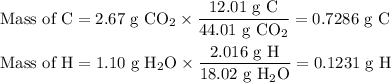
2. Calculate the moles of each element
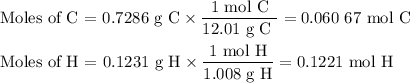
3. Calculate the molar ratios
Divide all moles by the smallest number of moles.
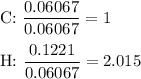
4. Round the ratios to the nearest integer
C:H = 1:2
5. Write the empirical formula
The empirical formula is