Answer:
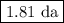
Step-by-step explanation:
1. Calculate the decay constant
The integrated rate law for radioactive decay is 1
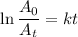
where
A₀ and A_t are the counts at t = 0 and t
k is the radioactive decay constant
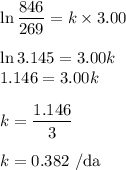
2. Calculate the half-life
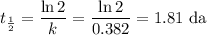
The half-life for decay is
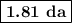 .
.