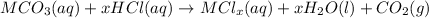Answer: metal and carbonate
Explanation:
According to Arrhenius concept, a base is defined as a substance which donates hydroxide ions
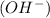 when dissolved in water and an acid is defined as a substance which donates hydrogen ions
when dissolved in water and an acid is defined as a substance which donates hydrogen ions
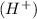 in water.
in water.
An acid is represented as :
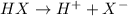
1. When metal is treated with an acid such as
 , if the metal is more reactive than hydrogen displaces hydrogen from its salt solution and thus produce zinc chloride and hydrogen gas.
, if the metal is more reactive than hydrogen displaces hydrogen from its salt solution and thus produce zinc chloride and hydrogen gas.
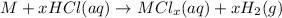
2. When carbonates are treated with acid, double displacement takes place ad carbon dioxide is released as a gas.