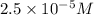Answer : The concentration of hydroxide and hydronium ion is,
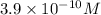 and
and
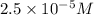
Explanation: Given,
pH = 4.6
pH : It is defined as the negative logarithm of hydrogen ion and hydronium ion concentration.
![pH=-\log [H_3O^+]](https://img.qammunity.org/2020/formulas/chemistry/middle-school/xj6fwrpeduepfcp6k4cv1s827uf5c0pbp1.png)
First we have to calculate the
 concentration.
concentration.
![pH=-\log [H_3O^+]](https://img.qammunity.org/2020/formulas/chemistry/middle-school/xj6fwrpeduepfcp6k4cv1s827uf5c0pbp1.png)
![4.6=-\log [H_3O^+]](https://img.qammunity.org/2020/formulas/chemistry/middle-school/l2k0htu4u7f7yf93cxg51oo3ahxuwf8zpp.png)
![[H_3O^+]=2.5* 10^(-5)M](https://img.qammunity.org/2020/formulas/chemistry/middle-school/tjr424cptfmnhtaur6f1g9zsqz0fniy7nx.png)
Now we have to calculate the pOH.
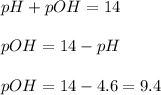
Now we have to calculate the
 concentration.
concentration.
![pOH=-\log [OH^-]](https://img.qammunity.org/2020/formulas/chemistry/high-school/h1t4ubcsdqvqg0xpalkkvnwrun04y9pzd8.png)
![9.4=-\log [OH^-]](https://img.qammunity.org/2020/formulas/chemistry/middle-school/ksgwdh75miiphgf8ijn6wl1uxzonxt0lui.png)
![[OH^-]=3.9* 10^(-10)M](https://img.qammunity.org/2020/formulas/chemistry/middle-school/hl4zor588wyjxrvuowhb42casisxu20i4r.png)
Therefore, the concentration of hydroxide and hydronium ion is,
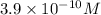 and
and