Answer: 24.87g Al2O3
Explanation: Aluminum is our limiting reagent
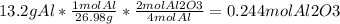
First of all we need to convert the grams of aluminium to moles, then use the molar fraction of the balanced equation (4 moles of aluminium equals 2 of aluminum oxide).
If we made the same procedure with the oxigen we get a 0.273 mol of Al2O3, therefore the O2 is the excess reagent.
The last step is convert the moles of the limiting reagent to grams
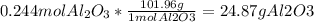
And that´s it!