Answer:
1.99 grams of methane
Step-by-step explanation:
A combustion reaction always produces the product carbon dioxide and water. So the chemical equation for this reaction would be:
CH₄ + O₂ → CO₂ + H₂O
To answer this question, you need to first determine which is the excess reactant. First step is to balance the equation:
CH₄ + 2O₂ → CO₂ + 2H₂O
Next we get the number of moles of each reactant we actually have:
8.00 g of O₂ = ? moles of O₂
You can get the number of moles, by first computing how many grams of the molecule is present in 1 mole.
O = 15.999g/mole. Since there are 2 oxygens in one mole of O₂, all you need to do is add up the atomic mass of 2 oxygens. You will get:
O₂= 31.998 g/mole
You can use this then to determine how many moles of O₂ there are in 8.00g.
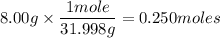
So there are 0.250 moles of O₂ in 8.00 g of O₂.
We do the same for CH₄
4.00g of CH₄=? moles of CH₄
C H₄
CH₄= 12.011 + 1.008(4) = 16.043 g/mole
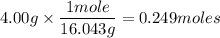
So let's sum up our new given. We now have:
0.250 moles of O₂
0.249 moles of CH₄
Next we look at the molar ratio of reactants to produce products:
CH₄ + 2O₂ → CO₂ + 2H₂O
According to this equation we can assume the following:
We need 1 mole of CH₄ for every 2 moles of O₂ in this reaction. Using what we have, we will see how much of reactant of the other reactant we need to use up the other.
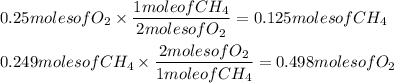
Compare the results with what we have:
What we have What we need
0.250 moles of O₂ < 0.498 moles of O₂
0.249 moles of CH₄ > 0.125 moles of CH₄
This means that since we have less O₂ that what we need to use up CH₄, then O₂ is the limiting reactant and CH₄ is the excess.
To compute how much we have in excess, we use the number of moles produced when we use up limiting reactant which we did earlier and convert it into grams to determine how much in grams we used up.
Earlier we solved that we need 0.125 moles of CH₄ to use up all the O₂. Now convert that value into grams:
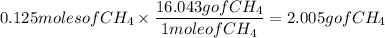
This means that 2.005g of CH₄ will be used up.
Subtract that from the CH₄ we already have:
4.00 g - 2.005 g =1.99 g of CH₄