Answer:
At t = 2 minutes, remaining quantity of the radioactive element is 12.5 mg.
Explanation:
To get the answer of this question we will solve this further with the help of the equation
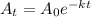
where k = decay constant
t = time for decay
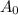 = Initial quantity taken
= Initial quantity taken
From the graph attached we can say that 50 mg of a radioactive element remained half in 1 minute.
So the equation becomes
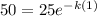
Now we take natural log on both the sides of the equation
ln50 = ln[25.
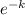
3.912 = ln25 +
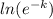
3.912 = 3.219 + (-k)lne
3.912 - 3.219 = -k [since lne = 1]
0.693 = -k
k = -0.693
Now we will calculate the remaining quantity of the element after 2 minutes
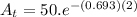
=
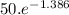
=
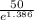
=
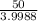
= 12.50 mg
Now we confirm this value from the graph.
At t = 2 minutes, remaining quantity of the radioactive element is 12.5 mg.