Answer:
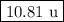
Step-by-step explanation:
The atomic mass of B is the weighted average of the atomic masses of its isotopes.
We multiply the atomic mass of each isotope by a number representing its relative importance (i.e., its percent of the total).
Set up a table for easy calculation:
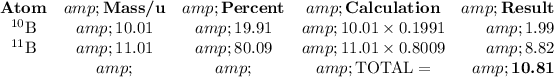
The average atomic mass of B is
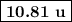 .
.