Answer : The wrong statement is, Two grams of hydrogen react with one gram of oxygen to form two grams of water.
Explanation :
Synthesis reaction : It is a type of reaction in which the reactants present in elemental state that reacts to give a single product.
It is represented as,
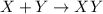
where, X and Y are the reactants and XY is the product.
The balanced synthesis reaction of water will be,
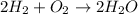
By the stoichiometry we can say that, 2 moles of hydrogen
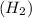 react with 1 mole of oxygen
react with 1 mole of oxygen
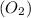 to form 2 moles of water
to form 2 moles of water
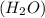 as a single product.
as a single product.
Or we can say that,
2 molecules of hydrogen react with 1 molecule of oxygen to form 2 molecules of water.
Or,
2 liters of hydrogen react with 1 liter of oxygen to form 2 liters of water.
These three statements are correct.
But the statement, 2 grams of hydrogen react with 1 gram of oxygen to form 2 grams of water are incorrect statement.
Hence, the wrong statement is, Two grams of hydrogen react with one gram of oxygen to form two grams of water.