Answer:
1. 2.8 moles of H₂
2. 7.38 moles of CO₂
3. 5.3 moles of O₂
4. 7.4 moles of KNO₃
Step-by-step explanation:
Here are the steps to doing this:
1. Write the chemical equation of each reaction.
2. Balance the equation.
3. Find out the ratio between reactant and product
4. Determine the actual yield of your reactants.
5. The amount of product produced is determined by how much product the limiting reactant produces.
Let's do this!
1. Given: 3.4 moles of Magnesium(Mg) and 5.6 moles of Hydrochloric acid (HCl)
Equation:
Mg + 2HCl → MgCl₂ + H₂
Reactant to Product ratio
1 mole of Mg produces 1 mole of H₂
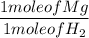
2 moles of HCl produces 1 mole of H₂
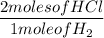
Determine actual yield of reactants
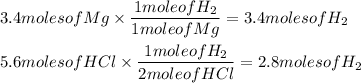
Since 5.6 moles of HCl can only produce 2.8 moles of H₂, before it is used up, then this means that that is all the product this reaction can produce.
2. Given: 3.4 moles of C₃H₈ and 12.3 moles of oxygen gas (O₂)
Equation:
C₃H₈ + 5O₂ → 3CO₂ + 4H₂O
Reactant to Product ratio
1 mole of C₃H₈ produces 3 moles of CO₂
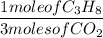
5 moles of O₂ produces 3 moles of CO₂
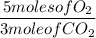
Determine actual yield of reactants
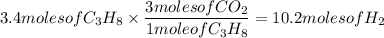
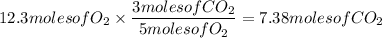
The answer is then 7.38 moles of CO₂
**3. 5.3 moles of H₂O
This one is a little bit different. It is asking how much of a reactant is needed to produce the amount of product given. For this, just write a balanced equation for the reaction and get the ratio of reactant to product and solve for the actual yield. Since it is only asking for oxygen gas, you just need to do that one.
Equation:
CH₄ + 2O₂ → CO₂ + 2H₂O
Reactant to Product ratio
![(2molesofO_(2))/(2molesofH_(2)O)=\[tex]7.88molesofKI*(1moleofKNO_(3))/(1moleofKI)=7.88molesofKNO_(3)](https://img.qammunity.org/2020/formulas/chemistry/middle-school/2fzs1y6p6tct3raei2ltohg5nz6cy5j1bl.png)
Actual yield:
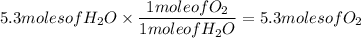
The answer is 5.3 moles of O₂.
4. 3.7 moles of Lead (II) Nitrate (Pb(NO₃)₂) and 7.8 moles of Potassium Iodide (KI)
Equation:
Pb(NO₃)₂ + 2KI → PbI₂ + 2KNO₃
Reactant to Product ratio
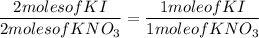
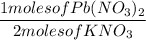
Actual yield:
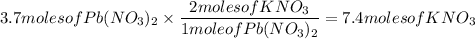
The answer is 7.4 moles of KNO₃.