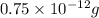Answer:
Step-by-step explanation:
Formula used :
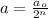
where,
a = amount of reactant left after n-half lives = ?
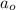 = Initial amount of the reactant =
= Initial amount of the reactant =
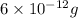
n = number of half lives = 3
Putting values in above equation, we get:
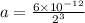
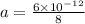
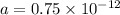
Therefore, the amount of carbon-14 left after 3 half lives will be