Answer:
There are 4 moles of sulfur atoms present in 2.00 moles of carbon sulfide.
Step-by-step explanation:
In 1 mole of carbon sulfide there are 1 mole of carbon atoms and 2 moles of sulfur atoms.
Then moles of carbon in 2 moles of carbon sulfide ;
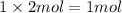 of carbon atom
of carbon atom
Then moles of sulfur in 2 moles of carbon sulfide ;
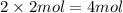 of sulfur atom
of sulfur atom
There are 4 moles of sulfur atoms present in 2.00 moles of carbon sulfide.