Answer:
Mass of 50 moles of tin(II) sulfate is 10,738 grams.
Step-by-step explanation:
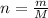
Where:
n = moles of compound
m = mass of compound
M = molar mass of the compound
Moles of tin(II) sulfate , n= 50 moles
Molar mass of tin (II) sulfate , M= 214.77 g/mol
m = n × M
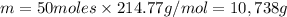
Mass of 50 moles of tin(II) sulfate is 10,738 grams.