Answer:
The balanced chemical reaction is:
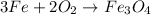
Step-by-step explanation:
Balanced chemical reaction : It is defined as the reaction in which the number of atoms of individual elements present on reactant side must be equal to the product side.
While balancing the chemical equation balance all the atoms of elements except oxygen atom which will be done in the last.
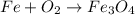
Step 1: Write three in front of iron;
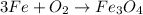
Step 2: Now write two in front of oxygen gas;
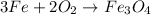
The balanced chemical reaction is:
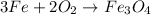
According to reaction, 3 moles of iron reacts with 2 moles of oxygen gas to give 1 mole of ferric oxide.