Answer:
Step-by-step explanation:
The tarnish on silverware is silver sulfide.
You can remove the tarnish by resting the silverware on a piece of aluminium foil in a pot of boiling water with a small amount of salt.
The reaction is an example of an electrochemical cell.
The half-cell reactions are
3×[Ag₂S(s) + 2e⁻ ⟶ 2Ag(s) + S²⁻(aq)]
2×[Al(s) ⟶ Al³⁺(aq) + 3e⁻]
2Al³⁺(aq) + 3S²⁻(aq) ⟶ Al₂S₃(s)
3Ag₂S(s) + 2Al(s) ⟶ 6Ag(s) + Al₂S₃(s)
The aluminium ions react with the sulfide ions to form aluminium sulfide.
The aluminum atoms lose three electrons each, so they are oxidized.
The silver atoms gain one electron each, so they are reduced.
Thus, the answer is
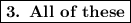 .
.