Answer:
Option (b) "inversely
"
Step-by-step explanation:
The energy of a photon is given by :
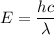
Where
h is the Planck's constant
c is the speed of light
 is the wavelength
is the wavelength
Or it can be written as :
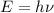 .....(1)
.....(1)
 is the frequency.
is the frequency.
Equation (1) can be rearranged as :
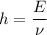
So, it is clear that Planck's constant relates the energy in one photon of electromagnetic radiation is inversely proportional to the frequency of the radiation. Hence, the correct option is (b) "inversely".