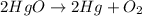Answer : The correct balanced equation is,
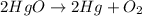
Explanation :
Balanced chemical equation : It is defined as the number of atoms of individual elements present on reactant side must be equal to the product side.
The balanced chemical reaction is,
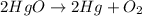
This chemical reaction is balanced reaction because in this reaction, the number of atoms are balanced on both the sides.
While the other equations are not balanced because the number of atoms are not balanced on both the sides.
Hence, the correct balanced equation is,