Answer:

Step-by-step explanation:
HCOOH + H₂O ⇌ H₃O⁺ + HCOO⁻
HCHO₂ is a weak acid. It dissociates only to a few percent, so there will be more HCHO₂ than H₃O⁺ present.
After H₂O, the most abundant species will be undissociated HCHO₂, so the answer will be either (B) or (D).
We can use an ICE table to organize the calculation of the pH.
HCOOH +H₂O ⇌ H₃O⁺ + HCOO⁻
I/mol·L⁻¹: 0.5 0 0
C/mol·L⁻¹: -x +x +x
E/mol·L⁻¹: 0.5 - x x x
![K_{\text{a}} = \frac{\text{[H}_(3)\text{O}^(+)]\text{HCOO}^(-)]} {\text{[HCOOH]}} = 2 * 10^(-4)\\\\(x^(2))/(0.5-x) = 2 * 10^(-4)](https://img.qammunity.org/2020/formulas/chemistry/high-school/jkgby1grs2gb0kqc2lcs5dy8n04dpfmn4m.png)
Check for negligibility of x
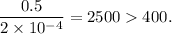
∴ x ≪ 0.5
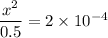
x² = 0.5 × 2 × 10⁴ = 1 × 10⁻⁴
x = √(1 × 10⁻⁴) = 1 × 10⁻²
[H₃O⁺] = x mol·L⁻¹ = 1 × 10⁻² mol·L⁻¹
pH = -log[H₃O⁺] = -log(1 × 10⁻²) = 2
The correct answer is
 .
.