Answer:
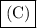
Step-by-step explanation:
The solid consists of positive and negative ions, so it is an ionic solid.
Ionic solids are brittle, usually water-soluble, and poor thermal conductors as solids. Thus, the correct answer is
 .
.
(A) is wrong. It describes a covalent solid, which is soft and always a poor conductor.
(B) is wrong. It describes a covalent solid, which is also low-melting.
(D) is wrong. It describes a metal, and a metal does not contain negative ions.