Answer:
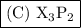
Step-by-step explanation:
Step 1. Identify the Group that contains X
We look at the consecutive ionization energies and hunt for a big jump between them
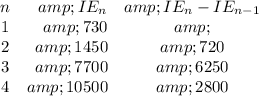
We see a big jump between n = 2 and n = 3. This indicates that X has two valence electrons.
We can easily remove two electrons, but the third electron requires much more energy. That electron must be in the stable, filled, inner core.
So, X is in Group 2 and P is in Group 15.
Step 2. Identify the Compound
X can lose two valence electrons to reach a stable octet, and P can do the same by gaining three electrons.
We must have 3 X atoms for every 2 P atoms.
The formula of the compound is
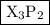 .
.