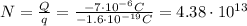Answer:
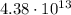 electrons
electrons
Step-by-step explanation:
The net charge on the sphere is equal to:
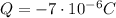
This charge is made up of N electrons. Each electron carries a charge of
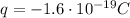
So the total charge is just the number of electrons times the charge of each electron:
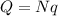
And solving the equation for N, we find the number of electrons: