Answer:

Step-by-step explanation:
There are three heat transfers in this process:
Total heat = warming the ice + melting the ice + warming the water
q = q₁ + q₂ + q₃
q = mC₁ΔT₁ + mΔHfus + mC₂ΔT₂
Let's calculate these heat transfers separately.
Data:
m = 1 kg = 1000 g
C₁ = 2.108 J·°C⁻¹g⁻¹
C₂ = 4.184 J·°C⁻¹g⁻¹
ΔHfus = 333 J/g
Tmax = 15 °C
m.p. = 0 °C
Tmin = -10 °C
Calculations:
ΔT₁ = 0 – (-10) = 10 °C
q₁ = 1000 × 2.108 × 10 = 21 080J = 21.08 kJ
q₂ = 1000 × 333 = 333 000 J =333 kJ
ΔT₂ = 15 - 0 = 15 °C
q₃ = 1000 × 4.184 × 15 = 62 760 J = 62.76 kJ
q = 21.08 + 333 + 62.76 = 417 kJ
You must add
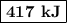 .
.