Answer:
Option D, the acidity of the lake to increase.
Step-by-step explanation:
The increase of hydrogen ion in lake water increases the acidity of the lake. The acidity of lake is associated with pH of the water which depends on the hydrogen ions concentration as established by the equation given below –
pH
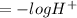
The hydrogen ion concentration is directly proportional to the acidity of the lake water. Thus, higher the H+ ion concentration, the higher will be the acidity of water.