Answer: The correct answer is Option D.
Step-by-step explanation:
A non-polar covalent bond is defined as the bond which is formed between the atoms having no difference in electronegativity values. For Example:
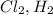 etc..
etc..
In this bond, the electrons are shared equally and
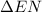 value is equal to 0.
value is equal to 0.
Hence, the correct answer is Option D.