Answer:
The theoretical yield of copper is approximately 1.65 grams.
Step-by-step explanation:
Relative atomic mass data from a modern periodic table:
- Al: 26.982;
- Cu: 63.546;
- Cl: 35.45.
What's the balanced equation for the reaction between
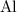 and
and
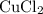 ?
?
As a metal, aluminum is more reactive than copper. Aluminum will reduce the
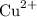 ion in
ion in
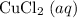 to
to
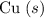 . This process will form a positive aluminum ion, which will then combine with chloride ions in the solution. What will be the charge on each aluminum ion? Aluminum is in IUPAC Group 13 of the periodic table. Each aluminum atom contains three valence electrons. As a main group metal in the p-block, each atom will lose all three of its valence electrons to form
. This process will form a positive aluminum ion, which will then combine with chloride ions in the solution. What will be the charge on each aluminum ion? Aluminum is in IUPAC Group 13 of the periodic table. Each aluminum atom contains three valence electrons. As a main group metal in the p-block, each atom will lose all three of its valence electrons to form
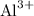 ions with three positive charges. Each ion will combine with three
ions with three positive charges. Each ion will combine with three
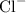 ions to produce a species with the empirical formula
ions to produce a species with the empirical formula
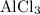 .
.
Reactants:
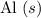 and
and
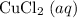 .
.
Products:
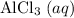 and
and
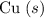 .
.
Let the coefficient in front of
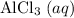 be
be
 .
.
![\begin{array}{cccccccl}\text{Al}\;(s) & +& \text{CuCl}_2\;(aq) & \to & \text{AlCl}_3\;(aq) & + & \text{Cu}\;(s)\\ & & & & {\bf 1} & & &\begin{aligned}&\text{Assign]()
![\begin{array}{cccccccl}\phantom{\;\text{Al}\;(s)} & \phantom{+}& \phantom{\text{CuCl}_2\;(aq)} & \phantom{\to} & \phantom{\text{AlCl}_3\;(aq)} & \phantom{+} & \phantom{\text{Cu}\;(s)}\\[-1em]1& &{3/2} & & 1 & & {\bf 3/2} &\begin{aligned}&\text{Cu atoms shall also}\\[-0.5em]&\text{conserve.}\end{aligned} \\2& &{3} & & 2 & & {3} &\begin{aligned}&\text{Multiply all}\\[-0.5em]&\text{coefficients by two}\\[-0.5em]&\text{to eliminate fractions.}\end{aligned}\end{array}]() .
.
Hence the balanced equation:
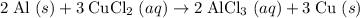 .
.
Which reactant is limiting?
Assume that
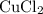 is the limiting reactant.
is the limiting reactant.
Formula mass of
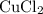 :
:
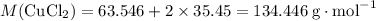 .
.
Number of moles of
 available:
available:
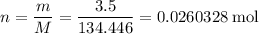 .
.
The ratio between the coefficient in front of
 is the same as the coefficient in front of
is the same as the coefficient in front of
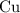 .
.
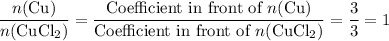 .
.
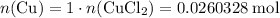 .
.
Mass of copper that is expected to be produced if
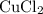 is the limiting reactant:
is the limiting reactant:
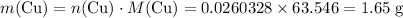 .
.
Assume that
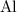 is the limiting reactant.
is the limiting reactant.
The methods are similar. Try the steps above yourself.
Formula mass of
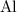 :
:
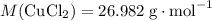 .
.
Number of moles of
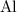 available:
available:
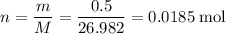 .
.
The ratio between the coefficient in front of
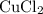 is
is
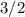 times the coefficient in front of
times the coefficient in front of
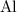 .
.
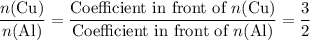 .
.
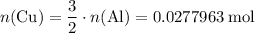 .
.
Mass of copper that is expected to be produced if
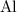 is the limiting reactant:
is the limiting reactant:
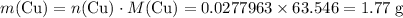 .
.
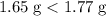 . The first assumption is valid.
. The first assumption is valid.
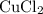 will run out before all
will run out before all
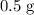 of aluminum are consumed, Only
of aluminum are consumed, Only
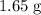 of copper will be produced.
of copper will be produced.