Answer:
An element may have several isotopes that contain different numbers of NEUTRONS but the same number of PROTONS.
Step-by-step explanation:
Isotopes are defined as the group of such atoms which will have same number of protons but different number of neutrons
So this is basically same atoms but with different number of neutrons
So here we can sat
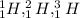
above are three isotopes of hydrogen
similarly we have many more examples of isotopes in which the atoms are of same type but with different number of neutrons
so here correct answer will be
An element may have several isotopes that contain different numbers of NEUTRONS but the same number of PROTONS.