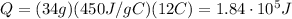Answer:
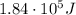
Step-by-step explanation:
The heat absorbed by the piece of iron is given by:
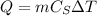
where:
m = 34 g is the mass of the piece of iron
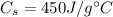 is the specific heat of iron
is the specific heat of iron
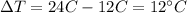 is the variation of temperature of the piece of iron
is the variation of temperature of the piece of iron
Substituting numbers into the formula, we find